What is the difference between light magnesium carbonate and heavy magnesium carbonate?
Light Magnesium Carbonate vs. Heavy Magnesium Carbonate: Seemingly Identical, Yet Vastly Different
In the world of chemistry, sometimes the subtlest differences can lead to drastically different applications. Magnesium carbonate (MgCO₃) is a prime example. When we talk about "magnesium carbonate," we usually refer to two physical forms:light magnesium carbonate and heavy magnesium carbonate. Although they share the same chemical formula and similar core chemical components, they differ significantly in physical properties, production processes, and end uses.
Core Difference: Physical Form and Density
As the names suggest, the most fundamental difference lies in their volume and density.
Light Magnesium Carbonate: Typically a very fluffy, light white powder. Its particles are small, with a large specific surface area and a loose, porous structure. Its bulk density is very low, typically between 0.1 - 0.3 g/cm³. Holding it in your hand, it feels like a wisp of smoke, easily dispersing.
Dense magnesium carbonate: This is a denser, heavier white powder or granules. The particles are larger, with a compact structure and a smaller specific surface area. Its bulk density is much higher than that of light magnesium carbonate, typically between 0.7 and 1.2 g/cm³ or higher. It feels more like ordinary table salt or flour.
A vivid analogy can be made: light magnesium carbonate is like freshly fallen, fluffy snow, while dense magnesium carbonate is like compacted, heavy, stale snow.
The root cause: Different production processes
This significant difference in physical form stems from their completely different manufacturing methods.
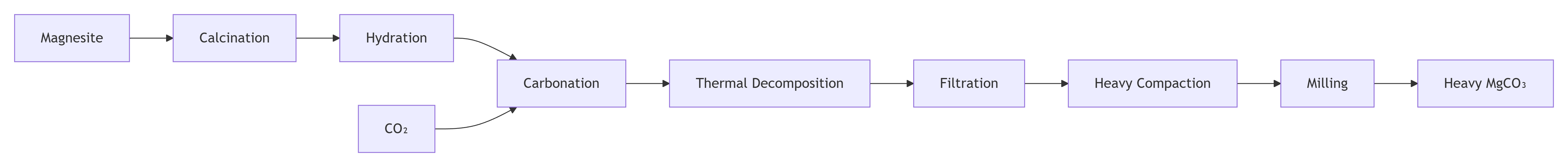
Light magnesium carbonate: Typically produced through chemical synthesis. The most common method involves calcining, digesting, and carbonizing magnesium-containing ores (such as dolomite) to generate a soluble magnesium salt solution. This solution is then reacted with sodium carbonate (Na₂CO₃) or directly precipitated with carbon dioxide to form basic magnesium carbonate (light magnesium carbonate typically exists as a basic salt,3MgCO₃·Mg(OH)₂·3H₂O). Finally, the solution is filtered, dried, and pulverized to obtain the final product. This process creates its porous and loose microstructure.

Heavy magnesium carbonate: Primarily obtained through the mechanical processing of the natural mineral magnesite (MgCO₃). The mined magnesite is crushed, ground, graded, and refined to remove impurities, yielding powder of the desired particle size. Because it originates from a natural mineral, its crystal structure is complete and dense. Alternatively, light magnesium carbonate can be converted to heavy magnesium carbonate under certain conditions through hydration.